The insect vector for T. brucei is the tsetse
fly. The parasite lives in the midgut of the fly (procyclic form),
whereupon it migrates to the salivary glands for injection to the
mammalian host on biting. The parasite lives within the bloodstream
(bloodstream form) where it can reinfect the fly vector after biting.
Later during a T. brucei infection the parasite may migrate to
other areas of the host. A T. brucei infection may be transferred
human to human via bodily fluid exchange, primarily blood transfer.
There are three different sub-species of T. brucei, which
cause different variants of trypanosomiasis.
- T. brucei gambiense - Causes slow onset chronic trypanosomiasis in humans. Most common in central and western Africa, where humans are thought to be the primary reservoir.[1]
- T. brucei rhodesiense - Causes fast onset acute trypanosomiasis in humans. Most common in southern and eastern Africa, where game animals and livestock are thought to be the primary reservoir.[1]
- T. brucei brucei - Causes animal African trypanosomiasis, along with several other species of trypanosoma. T. b. brucei is not human infective due to its susceptibility to lysis by human apolipoprotein L1.[2] However, as it shares many features with T. b. gambiense and T. b. rhodesiense (such as antigenic variation) it is used as a model for human infections in laboratory and animal studies.
The cell structure
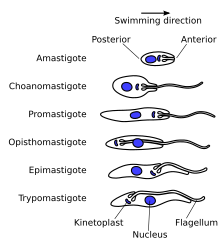
The structure of the cell is fairly typical of eukaryotes, see eukaryotic cell. All major organelles are seen, including the nucleus, mitochondria, endoplasmic reticulum, Golgi apparatus etc. Unusual features include the single large mitochondria with the mitochondrial DNA structure known as the kinetoplast, and its association with the basal body of the flagellum. The cytoskeleton is made up primarily of microtubules. The cell surface of the bloodstream form features a dense coat of variable surface glycoproteins (VSGs) which is replaced by an equally dense coat of procyclins when the parasite differentiates into the procylic in the tsetse fly midgut.- Epimastigote - Basal body anterior of nucleus, with a long flagellum attached along the cell body.
- Trypomastigote - Basal body posterior of nucleus, with a long flagellum attached along the cell body.
T. brucei is found as a trypomastigote in the slender, stumpy, procyclic and metacyclic forms. The procylic form differentiates to the proliferitive epimastigote form in the salivary glands of the insect. Unlike some other trypanosomatids, the promastigote and amastigote form do not form part of the T.brucei life cycle.
The genome
The genome of T. brucei is made up of[3]:- 11 pairs of large chromosomes of 1 to 6 megabase pairs.
- 3-5 intermediate chromosomes of 200 to 500 kilobase pairs.
- Around 100 mini chromosomes of around 50 to 100 kilobase pairs. These may be present in multiple copies per haploid genome.
The mitochondrial genome is found condensed into the kinetoplast, an unusual feature unique to the kinetoplastea class. It and the basal body of the flagellum are strongly associated via a cytoskeletal structure.
VSG surface coat
Main section: The VSG coatThe surface of the trypanosome is covered by a dense coat of Variable Surface Glycoprotein (VSG), which allows persistence of an infecting trypanosome population in the host. See below.
The cytoskeleton
The cytoskeleton is predominantly made up of microtubules, forming a subpellicular corset. The microtubules lie parallel to each other along the long axis of the cell, with the number of microtubules at any point roughly proportional to the circumference of the cell at that point. As the cell grows (including for mitosis) additional microtubules grow between the existing tubules, leading to semiconservative inheritance of the cytoskeleton. The microtubules are orientated + at the posterior and - at the anterior.Microfilament and intermediate filaments also play an important role in the cytoskeleton, but these are generally overlooked.
Flagellar structure
The trypanosome flagellum has two main structures. It is made up of a typical flagellar axoneme which lies parallel to the paraflagellar rod, a lattice structure of proteins unique to the kinetoplastida, euglenoids and dinoflagellates.The microtubules of the flagellar axoneme lie in the normal 9+2 arrangement, orientated with the + at the anterior end and the - in the basal body. The a cytoskeletal structure extends from the basal body to the kinetoplast. The flagellum is bound to the cytoskeleton of the main cell body by four specialised microtubules, which run parallel and in the same direction to the flagellar tubulin.
The flagellar function is twofold - locomotion via oscilations along the attached flagellum and cell body, and attachment to the fly gut during the procyclic phase.
The VSG coat
The surface of the trypanosome is covered by a dense coat of ~1x107 molecules of Variable Surface Glycoprotein (VSG).[4] This coat enables an infecting T. brucei population to persistently evade the host's immune system, allowing chronic infection. The two properties of the VSG coat that allow immune evasion are:- Shielding - the dense nature of the VSG coat prevents the immune system of the mammalian host from accessing the plasma membrane or any other invariant surface epitopes (such as ion channels, transporters, receptors etc.) of the parasite. The coat is uniform, made up of millions of copies of the same molecule; therefore the only parts of the trypanosome the immune system can 'see' are the N-terminal loops of the VSG that make up the coat.[5]
- Periodic antigenic variation - the VSG coat undergoes frequent stochastic genetic modification - 'switching' - allowing variants expressing a new VSG coat to escape the specific immune response raised against the previous coat.









0 komentar:
Posting Komentar